Electrochemical Analytical Laboratory
The Electrochemical Analytical Lab stands as a pivotal facility dedicated to assessing the corrosion behavior of a diverse range of conductive materials. These materials, processed/fabricated through innovative, non-conventional and additive methods such as Wire- Direct Energy Deposit (W-DED), thermal spray and friction stir welding, are studied under extreme conditions. This rigorous evaluation enables a comprehensive understanding of electrochemical degradation mechanisms, which is crucial for applications across various critical sectors, including marine, aerospace, automotive, and biomedical. Electrochemical studies not only enhances our grasp of corrosion mechanism in materials fabricated via non-conventional techniques but also drives the development of more durable and reliable materials for use in industry-specific applications, underlining the lab’s essential role in advancing material resilience and innovation.
Tools

Potentiostat Reference 3000
Potentiostats are indispensable in the electrochemical characterization of metals and alloys, providing precise control over electrochemical reactions crucial for understanding corrosion mechanisms. These instruments regulate the voltage between a working electrode and a reference electrode in an electrochemical cell, facilitating a broad range of tests including DC corrosion, pulse voltammetry and physical electrochemistry. This capability is essential for assessing the corrosion behavior of metals and alloys under extreme conditions, thereby informing the development of materials that are resistant to such environments.
Features/Specifications:
- Maximum Current ±3 A or ±1.5 A @ 32V
- Current Ranges 11 (300 pA – 3 A)
- Maximum Current Resolution 92 aA
- Maximum Applied Potential ±32 v
- Applied Accuracy ±1 mV ±0.2% of setting
- Applied Resolution 12.5 μV, 50 μV, 200 μV/bit

MultiPort Corrosion Cell Kit
The MultiPort Corrosion Cell Kit provides a versatile solution for corrosion testing in laboratory settings, ensuring reliable results through its two-piece construction. Designed to accommodate 1 L standards testing, it offers flexibility for various electrode setups and experimental requirements. Its compatibility with Gamry’s SCE, Ag/AgCl, and Hg/Hg2SO4 reference electrodes enables a range of AC and DC electrochemical tests. The cell accommodates a working volume between 400 mL and 1200 mL, making it ideal for comprehensive corrosion studies for varying sample size.
EuroCell Electrochemical Corrosion Cell Kit
The EuroCell corrosion cell Kit is designed for corrosion testing in smaller volumes, offering precise measurements and diverse electrochemical experimentation capabilities. With an operational volume ranging between 50-200 mL, this kit is equipped with 5 multi-purpose ports to accommodate various electrode setups. The EuroCell facilitates vertical adjustment for precise electrode positioning, ensuring accuracy in results. The EuroCell supports a range of reference electrodes such as SCE (Saturated Calomel Electrode), Ag/AgCl (Silver/Silver Chloride), and others, providing flexibility for various corrosion tests including potentiodynamic and cyclic polarization tests

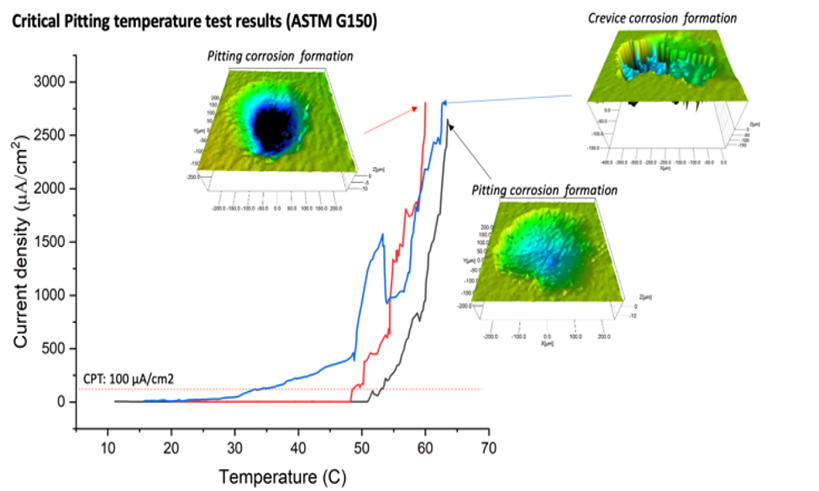
Critical Pitting Temperature Test
The Critical Pitting Temperature (CPT) serves as a pivotal parameter for evaluating and ranking metals and alloys
based on their susceptibility to pitting corrosion. It specifically denotes the lowest temperature at which stable pit
propagation occurs, offering a quantifiable measure to assess the resilience of materials against such localized
corrosion phenomena. This criterion is instrumental in identifying materials best suited for applications where pitting
corrosion resistance is critical, thereby guiding material selection and engineering decisions in environments prone to
aggressive corrosion. The main components of the test are:
Features/Specifications:
- Temperature Range: -25°C to 140°C
- Chemical Compatibility: Wetted Materials Pyrex®, PTFE, Filter Paper
- Accuracy: +/-0.5°C, 0.03% reading process
- Temperature Stability: w/ RTD = 0.04°C/°C, w/ TC = 0.05°C/°C

Salt Spray Fog Chamber
The Salt Spray Test is a critical accelerated corrosion assessment used to determine the resilience of a wide range of
materials, including metals, alloys, ceramics, stones, and polymers—whether coated or uncoated—against corrosive
environmental influences at high temperature. This test is integral for gauging the corrosion resistance of materials
fabricated via non-traditional manufacturing methods, particularly under the severe conditions of high salinity and
temperature typical of marine environments. Such testing is vital to comprehend how these materials perform and
endure in the face of real-world climatic conditions.
Features/Specifications:
- Adjustable Temperature Range: Room Temperature +5°C- 50 °C
- Temperature Accuracy: ≤ 0.5°C
- Homogeneous Chamber Temperature: ≤ 2°C

Tribocorrosion Set Up
Tribocorrosion is a surface degradation phenomenon resulting from the synergistic effects of both mechanical wear and electrochemical corrosion in a corrosive environment. Tribocorrosion testing involves conducting wear and corrosion experiments simultaneously using a sliding ball-on-disk setup, where the contact area undergoing wear is fully immersed in the test electrolyte. This setup includes a tribocorrosion module, which consists of a customized 3D printed Nylon cup and a three-electrodes system (Gamry Potentiostat) mounted on the modular tribometer stage. The sample serves as the working electrode connected through an insulated copper wire, graphite rod acts as the counter electrode, and a Calomel, NaCl (saturated) electrode functions as the reference electrode. The open circuit potential (OCP) is continuously measured in situ to monitor the progression of the tribocorrosion process, with a reduction in OCP serving as an indicator of protective film remosion. Additionally, changes in the coefficient of friction (COF) offer insights into various stages of wear development. The wear tests is also conducted under anodic or cathodic polarization conditions to accelerate the corrosion process, allowing for the investigation of the contribution of the mechanical forces and the electrochemical reactions on the surface degradation.
Ongoing Research Projects
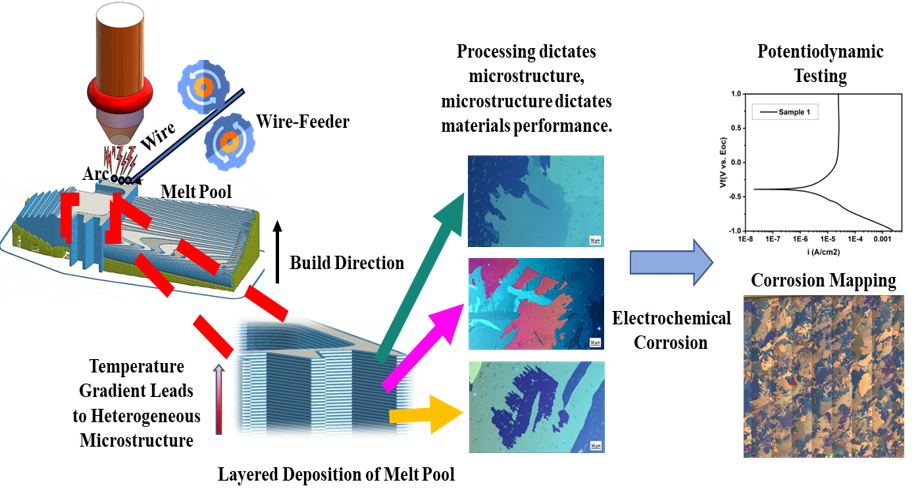
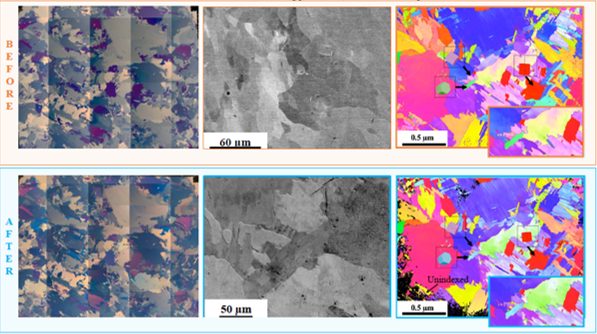
Grain Based Corrosion Mapping of Materials Fabricatedvia Wire Direct Energy Deposition (W-DED)
The Wire Direct Energy Deposition (W-DED) process introduces anisotropic grains primarily due to the directional solidification and thermal gradients inherent in the deposition process. The temperature gradient influences grain growth and orientation, leading to the formation of elongated grains parallel to the heat flow direction. The corrosion resistance of metallic components is heavily influenced by the microstructure, including grain size, orientation, and distribution. Grain-based corrosion mapping allows for the identification of regions susceptible to localized corrosion, providing valuable insights for corrosion mitigation strategies and material design. By characterizing the grain structure and correlating it with corrosion behavior, it becomes possible to tailor the microstructure of W-DED fabricated metals to optimize corrosion resistance. This knowledge can inform process parameter optimization, postprocessing treatments, or alloying strategies aimed at enhancing the material’s resistance to corrosive environments.
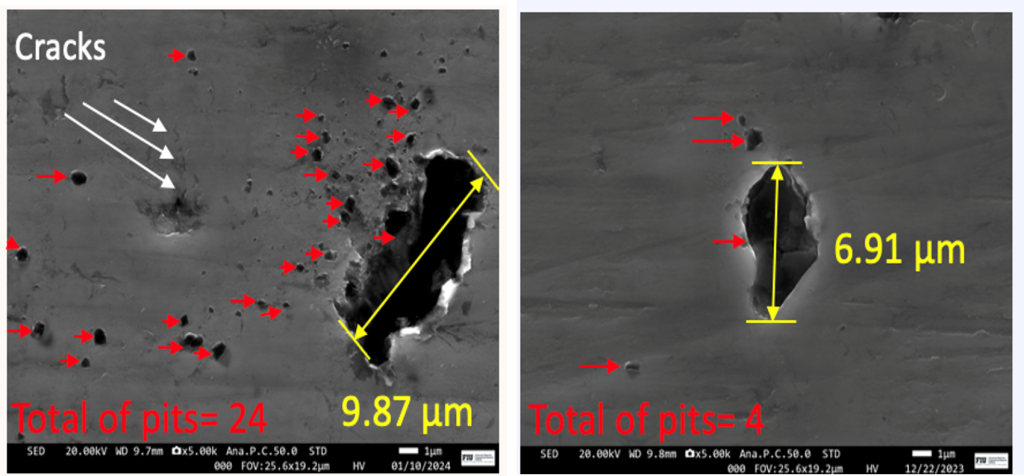
Critical Pitting Temperature of Materials Fabricatedvia Wire Direct Energy Deposition (W-DED)
The CPT test provides valuable information about the material’s resistance to localized corrosion, particularly insight into the temperature dependence of pitting corrosion to a specific environment. Given the critical nature of corrosion resistance in various industries, such as aerospace, marine, and chemical processing, understanding the CPT of W-DED commercially pure (CP) Ti is crucial for evaluating its suitability for specific applications where exposure to corrosive and high temperature environments is a concern. Comparing the CPT of W-DED CPTi with conventionally manufactured CP Ti provides insights into how the additive manufacturing process affects the material’s corrosion resistance as W-DED induces anisotropy in grain structure. Determining the CPT allows engineers and designers to establish safe operating temperature limits for W-DED CP Ti components in corrosive environments. Components operating above the CPT are at risk of localized corrosion initiation, leading to premature failure. Therefore, knowing the CPT helps mitigate corrosion-related risks and ensures the reliability and longevity of W-DED fabricated parts.