Atomic Force Microscopy
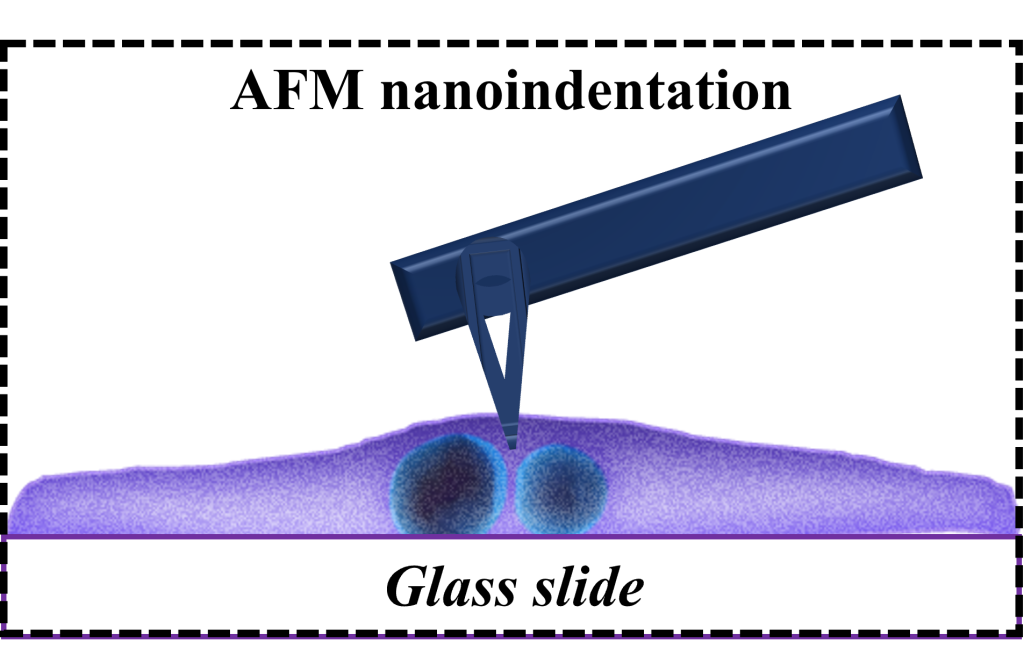
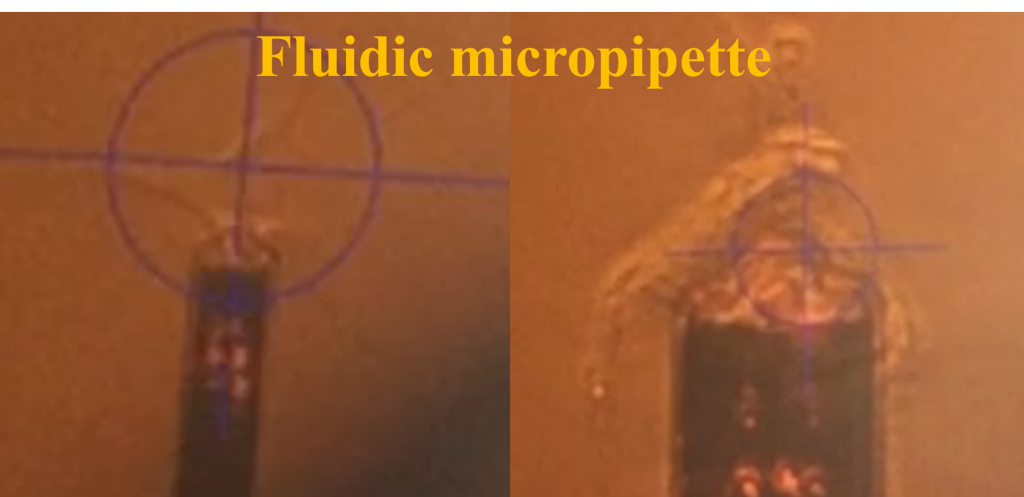
Atomic Force Microscopy (AFM) has been extensively utilized in the characterization of materials across zero-dimensional (0D), one-dimensional (1D), and two-dimensional (2D) constructs, as well as in the analysis of cellular structures. For 0D/1D/2D materials, our capabilities include the acquisition of topographical data, the assessment of surface electrical potential, and the mapping of mechanical moduli. In the realm of cellular characterization, we employ techniques such as nanoindentation under aqueous conditions and fluidic assays to quantify the forces pertinent to cell-substrate and cell-cell adhesion. Specifically focusing on cellular analysis, we have conducted studies to elucidate the spatiotemporal mechanical properties and viscoelastic behavior of myocytes. This aspect is of considerable interest as it is fundamentally involved in the maintenance of structural and functional equilibrium within cells. To investigate the time-dependent viscoelastic properties of cardiomyocytes, particularly within cross-linked polymeric networks, we have deployed AFM nanoindentation coupled with fluidic micropipette techniques to measure deformation and adhesion properties of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs).
Our empirical findings indicate that the cytoplasm of these cells withstands a load ranging from 7 to 14 nanonewtons, they exhibit a de-adhesion force from 0.1 to 1 nanonewton, and the adhesion force between two hiPSC-CMs lies between 50 to 100 nanonewtons, with an interfacial energy measurement of 0.45 picojoules. The load-displacement profiles obtained have been instrumental in modeling the dynamic viscoelastic behavior of these cells, revealing a strong correlation with their physiological attributes. Furthermore, our investigations into cell detachment and contractility suggest that the viscoelastic properties are pivotal in dictating the mechanics and function of hiPSC-CMs, especially in relation to cell-cell adhesion and strain during contractions. Collectively, our study furnishes critical insights into the interplay between structure, mechanics, and function in hiPSC-CMs, paving the way for refinements in single-cell mechanical testing platforms, which hold substantial promise for advancements in cellular biomechanics research.